

  |
 Using the catalytic power of enzymes to
perform highly stereoselective organic transformations has been the
basis of the ever-expanding field of biocatalysis. Enzymes have
evolved over millions of years to be some of the best catalysts
available to chemists. Now, through advances in molecular and
synthetic biology, chemists are able to redesign the active sights of
many enzymes to fit their synthetic needs.
Using the catalytic power of enzymes to
perform highly stereoselective organic transformations has been the
basis of the ever-expanding field of biocatalysis. Enzymes have
evolved over millions of years to be some of the best catalysts
available to chemists. Now, through advances in molecular and
synthetic biology, chemists are able to redesign the active sights of
many enzymes to fit their synthetic needs.
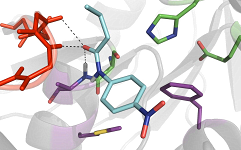
The image by U. Bornscheuer et al. is from an article on the activity of a bacillus subtilis esterase, which appeared in a special issue of ChemCatChem on biocatalysis.
Find all articles on biocatalysis in Wiley Online Library...